Solution: For nitrogen atom :
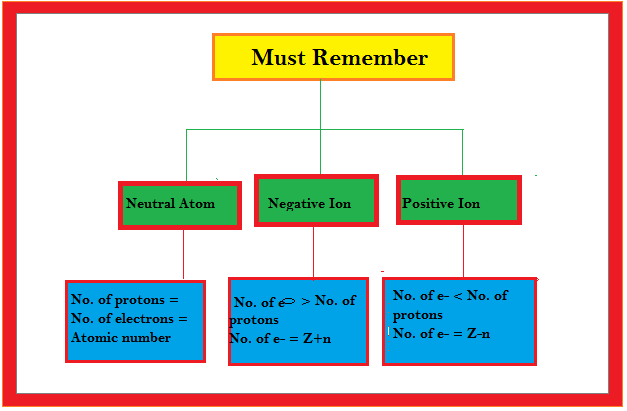
No. of electrons = 7 ( given )
No. of neutrons = 7 ( given)
`therefore` No. of protons `= Z = 7` ( `because` atom is electrically neutral).
Atomic number = `Z = 7`
Mass no. (A) = NO. of protons+ NO. of neutrons
` = 7+7 = 14`
For Calcium ion
No. of neutrons `= 20` ( given)
Atomic Number `(Z) = 20` ( given)
`therefore` No. of protons ` = Z = 20`
No. of electrons in calcium atom `= Z = 20`
But in the formation of calcium ion , two electrons are lost from the extranuclear part according to the equation `Ca → Ca^(2+) + 2e^(-)`
but the composition of the nucleus remains unchanged.
`therefore` No. of electrons in calcium ion `= 20-2 = 18`
Mass number(A) = No. of protons + No. of neutrons
` = 20+20= 40`
For oxygen atom :
Mass number (A) = No. of protons +No. of neutrons = 16 (given)
Atomic No. (Z) = 8.(given)
No. of protons `= Z = 8` (given)
No. of electrons `= Z = 8`
No. of neutrons `= A-Z = 16-8 = 8`
For bromide ion : In the formation of bromide ion one electron is added to the extra nuclear part of the bromine atom according to the equation `Br + e^(-) → Br^(-)` but the composition of the nucleus remains unchanged .
Since the no. of electrons in bromide ion = 36 (given)
No. of electrons in bromine atom ` = 36-1 = 35`
Further , since in the above reaction , nucleus of the bromine atom remains unchanged.
`therefore` No. of protons in the bromine ion = No. of protons in the bromine atom = 35.
Putting the values of the various vacant place as calculated above , in the table we have
| Particle | Mass Number (A) | Atomic Number (Z) | No. of Protons(Z) | No. of Neutrons(A-Z) | No. of Electrons(Z) |
|---|
| Nitrogen atom | 7+7 = 14 | 7 | 7 | 7 | |
| Calcium ion | 20+20= 40 | 20 | 20 | 20 | 20-2 = 18 |
| Oxygen atom | 16 | 8 | 8 | 16-8 = 8 | 8 |
| Bromide ion | 45+35 = 80 | 35 | 36-1 = 35 | 45 | 36 |